UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO SECTION 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of April, 2020
Commission File Number: 001-36815
Ascendis Pharma A/S
(Exact Name of Registrant as Specified in Its Charter)
Tuborg Boulevard 12
DK-2900 Hellerup
Denmark
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Spokespersons of Ascendis Pharma A/S (the “Company”) presented the information in the presentation slides attached hereto as Exhibit 99.1 in a webcast on April 19, 2020.
The furnishing of the attached presentation is not an admission as to the materiality of any information therein. The information contained in the presentation is summary information that is intended to be considered in the context of more complete information included in the Company’s filings with the Securities and Exchange Commission (the “SEC”) and other public announcements that the Company has made and may make from time to time. The Company undertakes no duty or obligation to update or revise the information contained in this report, although it may do so from time to time as its management believes is appropriate. Any such updating may be made through the filing or furnishing of other reports or documents with the SEC or through other public disclosures.
Exhibits
| 99.1 | Company Presentation | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Ascendis Pharma A/S | ||||||
| Date: April 20, 2020 | By: | /s/ Michael Wolff Jensen | ||||
| Michael Wolff Jensen | ||||||
| Chairman and Senior Vice President, Chief Legal Officer | ||||||

TransCon™ PTH Top-Line Phase 2 Data from PaTH Forward April 19, 2020 Exhibit 99.1

Cautionary Note On Forward-Looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, such as statements regarding our future results of operations and financial position, including our business strategy, prospective products, clinical trial results, product approvals and regulatory pathways, collaborations, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products, are forward-looking statements. These forward-looking statements are based on our current expectations and beliefs, as well as assumptions concerning future events. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the results discussed in the forward-looking statements. These risks, uncertainties and other factors are more fully described in our reports filed with or submitted to the Securities and Exchange Commission, including, without limitation, our most recent Annual Report on Form 20-F filed with the SEC on April 3, 2020 particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. In light of the significant uncertainties in our forward-looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. Any forward-looking statement made by us in this presentation speaks only as of the date of this presentation and represents our estimates and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these statements publicly, whether as a result of new information, future events or otherwise after the date of this presentation. This presentation concerns product candidates that are or have been under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency or other foreign regulatory authorities. These product candidates are currently limited by U.S. Federal law to investigational use, and no representations are made as to their safety or effectiveness for the purposes for which they are being investigated. Ascendis, Ascendis Pharma, the Ascendis Pharma logo, the company logo and TransCon are trademarks owned by the Ascendis Pharma group. ©April 2020 Ascendis Pharma A/S.
PaTH Forward Top-Line Data from 4-Week Fixed Dose Period PaTH Forward top-line data support TransCon PTH as a potential replacement therapy for adult HP TransCon PTH eliminated standard of care (i.e. off active vitamin D and ≤ 500 mg per day of calcium supplements) in 100% of subjects in the 21 µg/day arm and in 82% of subjects across all dosage arms Both the 21 µg/day arm and the combined TransCon PTH dosage arms showed a statistically significant response for the primary endpoint compared to placebo at 4 weeks TransCon PTH increased mean serum calcium TransCon PTH reduced mean urinary calcium excretion TransCon PTH reduced mean serum phosphate and calcium-phosphate product All doses of TransCon PTH were well-tolerated No serious or severe adverse events at any point No treatment-emergent adverse events (TEAEs) led to discontinuation of study drug Overall incidence of TEAEs comparable between TransCon PTH and placebo No drop-outs in blinded period

TransCon PTH Phase 2 Trial Design * PRO = patient-reported outcome ~40 adult subjects with HP currently receiving standard of care (active vitamin D + calcium) Primary Composite Endpoint (4 weeks) Proportion of subjects with: Normal serum calcium; and Normal FECa (or at least 50% decrease from baseline); and Off active vitamin D; and Taking ≤1,000 mg/day calcium supplements Blinded Treatment (4 weeks) RANDOMIZATION Screening ≤4 weeks Open-Label Extension TransCon PTH Individual Dosing (6 – 30 µg/day) TransCon PTH Titration & SoC Optimization Stable Dosing TransCon PTH 15 µg/day TransCon PTH 18 µg/day TransCon PTH 21 µg/day Placebo Key Secondary Composite Endpoint (4 weeks) Primary composite and taking ≤500 mg/day calcium supplements Additional Endpoints ≥4 weeks PRO* measures (HPES: a disease-specific PRO for HP) Nephrolithiasis, nephrocalcinosis, vascular calcification, ER/urgent care visits and hospitalizations BMD and TBS by DXA, bone turnover markers, 24-hour urine calcium excretion (in extension only) ALL SUBJECTS
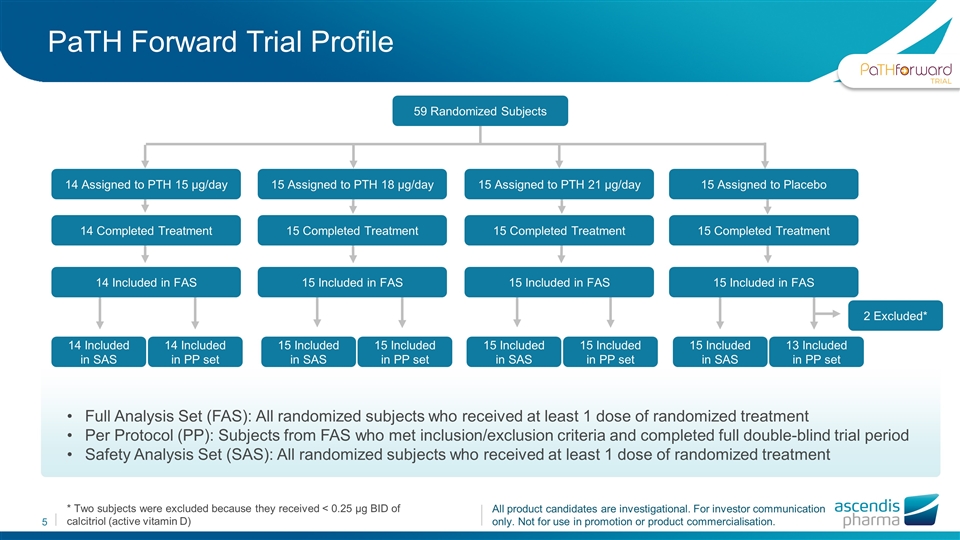
* Two subjects were excluded because they received < 0.25 µg BID of calcitriol (active vitamin D) PaTH Forward Trial Profile 59 Randomized Subjects 14 Assigned to PTH 15 µg/day 14 Completed Treatment 14 Included in FAS 15 Assigned to PTH 18 µg/day 15 Completed Treatment 15 Included in FAS 15 Assigned to PTH 21 µg/day 15 Completed Treatment 15 Included in FAS 15 Assigned to Placebo 15 Completed Treatment 15 Included in FAS 14 Included in SAS 14 Included in PP set 15 Included in SAS 15 Included in PP set 15 Included in SAS 15 Included in PP set 15 Included in SAS 13 Included in PP set 2 Excluded* Full Analysis Set (FAS): All randomized subjects who received at least 1 dose of randomized treatment Per Protocol (PP): Subjects from FAS who met inclusion/exclusion criteria and completed full double-blind trial period Safety Analysis Set (SAS): All randomized subjects who received at least 1 dose of randomized treatment

Demographics and Baseline Characteristics – PP PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Age (years) (n) 14 15 15 44 13 Mean (SD) 47 (13) 47 (11) 54 (11) 49 (12) 50 (13) Age Group (years) – n (%) < 30 1 (7.1) 1 (6.7) 0 2 (4.5) 1 (7.7) ≥ 30 - < 65 11 (79) 14 (93) 13 (87) 38 (86) 11 (85) ≥ 65 2 (14) 0 2 (13) 4 (9.1) 1 (7.7) Sex at Birth n (%) Female 12 (86) 12 (80) 12 (80) 36 (82) 10 (77) Body Mass Index (kg/m2) (n) 14 15 15 44 13 Mean (SD) 27 (5.7) 29 (3.1) 26 (4.6) 27 (4.6) 28 (3.8) Menopausal Status – n (%) 12 12 12 36 10 Postmenopausal 4 (33) 4 (33) 5 (42) 13 (36) 3 (30)

Demographics and Baseline Characteristics – PP PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Race – n (%) American Indian or Alaska Native 0 0 0 0 0 Asian 0 0 2 (13) 2 (4.5) 0 Black or African American 0 0 0 0 0 Native Hawaiian or Other Pacific Islander 0 0 0 0 0 White 14 (100) 12 (80) 13 (87) 39 (89) 13 (100) Unknown 0 0 0 0 0 Other 0 3 (20) 0 3 (6.8) 0 Geographic Region – n (%) North America 7 (50) 12 (80) 10 (67) 29 (66) 7 (54) Europe 7 (50) 3 (20) 5 (33) 15 (34) 6 (46)

HP Disease Characteristics and History – PP PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Cause of Hypoparathyroidism (HP) Acquired from neck surgery 10 (71) 12 (80) 12 (80) 34 (77) 11 (85) Autoimmune disease 1 (7.1) 0 0 1 (2.3) 0 Idiopathic disease 3 (21) 3 (20) 3 (20) 9 (20) 2 (15) Duration of HP (Years) (n) 14 15 15 44 13 Mean 12 9.3 12 11 13 Min, Max 1, 39 2, 29 3, 25 1, 39 3, 30 Renal Insufficiency History 1 (7.1) 3 (20) 1 (6.7) 5 (11) 0 Kidney Stones History 2 (14) 1 (6.7) 1 (6.7) 4 (9.1) 4 (31) Ectopic Calcifications History 0 0 1 (6.7) 1 (2.3) 0 Vascular Calcifications History 0 0 0 0 0 Brain Calcification History 0 0 0 0 0 Cataract History 0 0 0 0 0 Seizure History 1 (7.1) 0 0 1 (2.3) 1 (7.7)

Baseline HP Supplements – PP 2 subjects did not have eDiary information confirmed by prescription information HP Supplements at Baseline collected by eDiary/ Total Daily Dose (TDD) PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Calcium /TDD (mg) (n) 14 14 15 43 13 Mean 1643 2395 2334 2129 1636 Min, Max 500, 4000 900, 8000 500, 4500 500, 8000 800, 3200 Calcium Category, n (%) ≤ 2000 mg TDD 11 (79) 9 (60) 6 (40) 26 (59) 9 (69) > 2000 mg TDD 3 (21) 5 (33) 9 (60) 17 (39) 4 (31) Calcitriol (Active Vitamin D) /TDD (µg) (n) 10 11 13 34 8 Mean 1.025 0.750 0.750 0.831 0.719 Min, Max 0.50, 3.00 0.50, 1.25 0.50, 2.00 0.50, 3.00 0.50, 1.00 Alfacalcidol (Active Vitamin D) /TDD (µg) (n) 4 3 2 9 4 Mean 2.75 2.00 2.00 2.33 2.50 Min, Max 2.0, 4.0 1.0, 3.0 1.0, 3.0 1.0, 4.0 1.0, 4.0

Baseline of Spot FECa & Albumin-Adjusted sCa – PP Lab Summary at Baseline PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Albumin-Adjusted sCa (mg/dL) (n) 14 15 15 44 13 Mean (SD) 8.6 (0.49) 9.1 (1.3) 8.7 (0.62) 8.8 (0.91) 8.9 (0.39) Spot AM FECa (%) (n) 14 15 15 44 13 Mean (SD) 2.5 (1.4) 3.3 (1.5) 2.4(1.2) 2.8 (1.4) 2.3 (0.76) Spot AM FECa normal (≤ 2%) at baseline 7 (50%) 4 (27%) 8 (53%) 19 (43%) 5 (39%)

Treatment-Emergent Adverse Event Summary – SAS * Subjects are counted only in the highest severity category PTH 15 µg/day (N=14) n (%) PTH 18 µg/day (N=15) n (%) PTH 21 µg/day (N=15) n (%) Total PTH (N=44) n (%) Placebo (N=15) n (%) TEAEs 6 (43) 3 (20) 8 (53) 17 (39) 5 (33) Serious TEAE 0 0 0 0 0 Severity* Severe TEAE 0 0 0 0 0 Moderate TEAE 1 (7.1) 1 (6.7) 1 (6.7) 3 (6.8) 3 (20) Mild TEAE 5 (36) 2 (13) 7 (47) 14 (32) 2 (13) Related TEAE 3 (21) 1 (6.7) 5 (33) 9 (20) 1 (6.7) Serious Related TEAE 0 0 0 0 0 TEAE Related to Hyper- or Hypocalcaemia Leading to ER/Urgent Care Visit and/or Hospitalization 0 0 0 0 0 TEAE Leading to Discontinuation of Study Drug 0 0 0 0 0 TEAE Leading to Discontinuation of Trial 0 0 0 0 0 TEAE Leading to Death 0 0 0 0 0

Treatment-Emergent Adverse Events of Interest – SAS Includes ≥ 2 subjects in the PTH group or hyper- or hypocalcaemia Preferred Term PTH 15 µg/day (N=14) n (%) PTH 18 µg/day (N=15) n (%) PTH 21 µg/day (N=15) n (%) Total PTH (N=44) n (%) Placebo (N=15) n (%) TEAEs 6 (43) 3 (20) 8 (53) 17 (39) 5 (33) Headache 3 (21) 1 (6.7) 2 (13) 6 (14) 0 Nausea 2 (14) 1 (6.7) 1 (6.7) 4 (9.1) 1 (6.7) Fatigue 0 1 (6.7) 1 (6.7) 2 (4.5) 0 Injection site haemorrhage 1 (7.1) 0 1 (6.7) 2 (4.5) 0 Injection site pain 1 (7.1) 0 1 (6.7) 2 (4.5) 0 Thirst 0 1 (6.7) 1 (6.7) 2 (4.5) 0 Urinary tract infection 1 (7.1) 0 1 (6.7) 2 (4.5) 0 Hypertension 1 (7.1) 1 (6.7) 0 2 (4.5) 0 Hypercalcaemia 0 0 2 (13) 2 (4.5) 0 Hypocalcaemia 0 0 0 0 1 (6.7)

PaTH Forward 4-Week Fixed Dose Safety Summary All doses of TransCon PTH were well-tolerated No drop-outs during 4-week blinded period No serious or severe TEAEs were reported No TEAEs leading to discontinuation of study drug Overall incidence of TEAEs comparable between TransCon PTH and placebo TEAEs in TransCon arms reflect known PTH pharmacology Injections were well-tolerated using pen injector planned for commercial presentation Titration algorithm to eliminate standard of care demonstrated no hypocalcaemic AEs

Elimination of Standard of Care – PP * Not taking active vitamin D and ≤500 mg/day of calcium supplements Number of Subjects Meeting Each Component PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Not taking active vitamin D supplements 14 (100%) 14 (93%) 15 (100%) 43 (98%) 4 (31%) Taking ≤1000 mg/day of calcium supplements 13 (93%) 13 (87%) 15 (100%) 41 (93%) 6 (46%) Taking ≤500 mg/day of calcium supplements 12 (86%) 9 (60%) 15 (100%) 36 (82%) 2 (15%) Taking 0 mg/day of calcium supplements 7 (50%) 7 (47%) 8 (53%) 22 (50%) 0 Not taking active vitamin D and 0 mg/day of calcium supplements 7 (50%) 7 (47%) 8 (53%) 22 (50%) 0 Not taking active vitamin D and ≤500 mg/day of calcium supplements 12 (86%) 9 (60%) 15 (100%) 36 (82%) 2 (15%) 100% of subjects in the 21 µg/day arm and 82% of all subjects across all TransCon PTH dosage arms were able to eliminate standard of care*

Mean Active Vitamin D Dose by Visit – PP TransCon PTH enabled discontinuation of active vitamin D at week 2 Mean Active Vitamin D Dose ± SE (µg/day)

Mean Calcium Supplement Dose by Visit – PP TransCon PTH enabled continuous calcium supplement reduction over 4-week study period Mean Calcium Dose ± SE (mg/day)

Mean Serum Calcium and Spot FECa by Visit – PP TransCon PTH subjects exhibited reduced FECa, despite increased serum calcium For placebo subjects, FECa followed serum calcium levels Mean Calcium Corrected for Albumin ± SE (mg/dL) Mean Fractional Excretion of Calcium ± SE (%) Mean Serum Calcium Mean Spot FECa

TransCon PTH Increased Number of FECa Responders – PP By week 4 of treatment, TransCon PTH had normalized an additional 8 subjects compared to none on placebo % of Subjects with Normal AM FECa Baseline Week 4 Baseline Week 4 Baseline Week 4 Baseline Week 4

Mean Serum Phosphate by Visit – PP TransCon PTH subjects demonstrated consistent, sustained reductions in serum phosphate Mean Serum Phosphate ± SE (mg/dL)
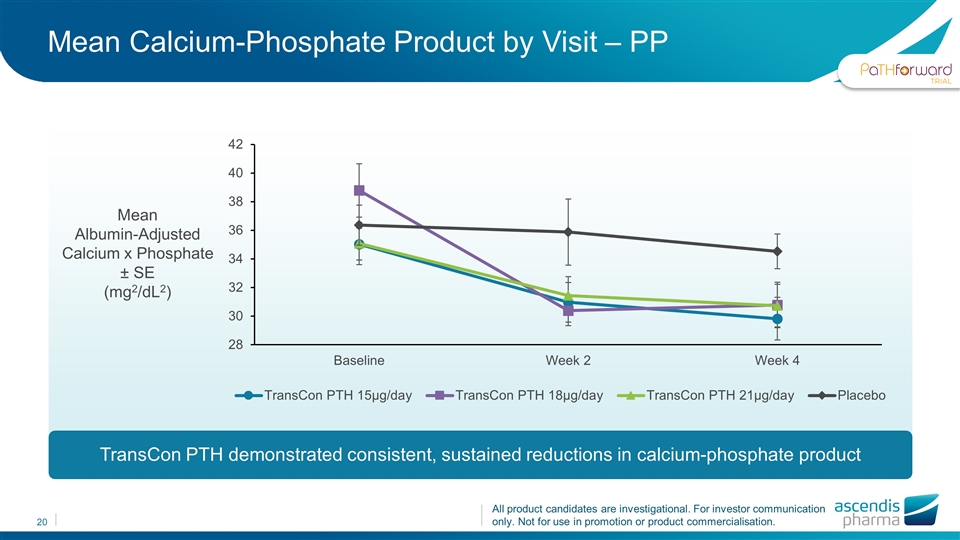
Mean Calcium-Phosphate Product by Visit – PP TransCon PTH demonstrated consistent, sustained reductions in calcium-phosphate product Mean Albumin-Adjusted Calcium x Phosphate ± SE (mg2/dL2)

Primary Composite Endpoint at Week 4 – PP PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Number of Subjects Meeting Primary Composite Endpoint at Week 4 with Fixed Dosing 7 6 9 22 2 Proportion (95% CI) 50 (23, 77) 40 (16, 68) 60 (32, 84) 50 (35, 65) 15 (1.9, 45) P-value 0.10 0.22 0.02 0.03 Number of Subjects Meeting Each Component: Serum calcium within the normal range, n (%) 12 (86%) 12 (80%) 14 (93%) 38 (86%) 12 (92%) Below lower limit of normal (<8.3 mg/dL) 2 1 0 3 1 Above upper limit of normal (>10.6 mg/dL) 0 2 1 3 0 Spot AM FECa within normal range (≤2%) or a reduction by at least 50% from baseline, n (%) 10 (71%) 8 (53%) 9 (60%) 27 (61%) 5 (38%) Not taking active vitamin D supplements, n (%) 14 (100%) 14 (93%) 15 (100%) 43 (98%) 4 (31%) Taking ≤1000 mg/day of calcium supplements, n (%) 13 (93%) 13 (87%) 15 (100%) 41 (93%) 6 (46%) The 21 µg/day arm and the combined TransCon PTH dosage arms showed a statistically significant response compared to placebo at week 4

Key Secondary Composite Endpoint at Week 4 – PP PTH 15 µg/day (N=14) PTH 18 µg/day (N=15) PTH 21 µg/day (N=15) Total PTH Subjects (N=44) Placebo (N=13) Number of Subjects Meeting Key Secondary Composite Endpoint at Week 4 with Fixed Dosing 7 4 9 20 2 Proportion (95% CI) 50 (23, 77) 27 (7.8, 55) 60 (32, 84) 45 (30, 61) 15 (1.9, 45) P-value 0.10 0.65 0.02 0.06 Number of Subjects Meeting Each Component: Serum calcium within the normal range, n (%) 12 (86%) 12 (80%) 14 (93%) 38 (86%) 12 (92%) Below lower limit of normal (<8.3 mg/dL) 2 1 0 3 1 Above upper limit of normal (>10.6 mg/dL) 0 2 1 3 0 Spot AM FECa within normal range (≤2%) or a reduction by at least 50% from baseline, n (%) 10 (71%) 8 (53%) 9 (60%) 27 (61%) 5 (38%) Not taking active vitamin D supplements 14 (100%) 14 (93%) 15 (100%) 43 (98%) 4 (31%) Taking ≤500 mg/day of calcium supplements 12 (86%) 9 (60%) 15 (100%) 36 (82%) 2 (15%) The 21 µg/day arm showed a statistically significant response compared to placebo at week 4

Key Takeaways from the PaTH Forward Trial – Primary Endpoint PaTH Forward Trial met the primary endpoint Overall statistical significance achieved notwithstanding: Short study duration of 4 weeks Fixed dose not individualized for each subject’s optimal dose Subjects continued to titrate off calcium supplements Small study population

Open-label Extension Trial Subjects from fixed-dose PaTH Forward Trial rolled over to the open-label extension which enabled individually optimized TransCon PTH dosing to evaluate long-term safety and efficacy 58 out of 59 randomized subjects currently receiving TransCon PTH in the open-label extension Both placebo responders continue in the open-label extension One subject (randomized to placebo) withdrew for reasons unrelated to safety or efficacy of the study drug Long-term data from open-label extension evaluates a composite endpoint. Evaluating proportion of subjects with: Normal serum calcium; and Off active vitamin D; and Taking ≤500 mg/day calcium; and Normal 24-hour urine calcium excretion (or at least 50% decrease from baseline)

Planned Next Steps Engage with global regulatory authorities on next steps for development of TransCon PTH Report PaTH Forward open-label extension six-month data in Q3 2020 Submit proposed PRO instrument for FDA review in Q3 2020 Submit regulatory filings to initiate a global phase 3 trial in North America, Europe and Asia in Q4 2020: Ethnobridging study showed comparable PK profile between Japanese and non-Japanese populations, enabling inclusion of Japan in global phase 3 program

PaTH Forward Top-Line Data from 4-Week Fixed Dose Period PaTH Forward top-line data support TransCon PTH as a potential replacement therapy for adult HP TransCon PTH eliminated standard of care (i.e. off active vitamin D and ≤ 500 mg per day of calcium supplements) in 100% of subjects in the 21 µg/day arm and in 82% of subjects across all dosage arms Both the 21 µg/day arm and the combined TransCon PTH dosage arms showed a statistically significant response for the primary endpoint compared to placebo at 4 weeks TransCon PTH increased mean serum calcium TransCon PTH reduced mean urinary calcium excretion TransCon PTH reduced mean serum phosphate and calcium-phosphate product All doses of TransCon PTH were well-tolerated No serious or severe adverse events at any point No treatment-emergent adverse events (TEAEs) led to discontinuation of study drug Overall incidence of TEAEs comparable between TransCon PTH and placebo No drop-outs in blinded period
